The Role of Genetics
It is becoming more common for ANE patients and their families to be tested for a RanBP2 variant. It is important to note, however, that even with the gene mutation, not all mutations will result in ANE. 40% of RanBP2 carriers will experience an episode of ANE after a viral illness. 50% of episodes occur before the age of 2 years.
The probability of recurrence after the first episode, is 50% and then 25% after a second episode. Note that triggers (preceding viral illness) vary from one patient to the next. Some patients will only have 1 trigger while others will have multiple.
RanBP2 Genetic Variations
RAN binding protein 2 is listed as a “major susceptibility factor in Familial Acute Necrotizing Encephalopthy” ORPHA:88619 on Orphanet, (portal for rare diseases and orphan drugs).
At least three mutations in the RANBP2 gene have been found to increase the risk of developing Acute Necrotizing Encephalopathy type 1 (ANE1). These mutations change single protein building blocks (amino acids) in the gene’s protein resulting in the production of a protein that cannot function properly. The mutations do not cause health issues on their own; it is not clear how they are involved in the process of a viral infection triggering neurological damage.
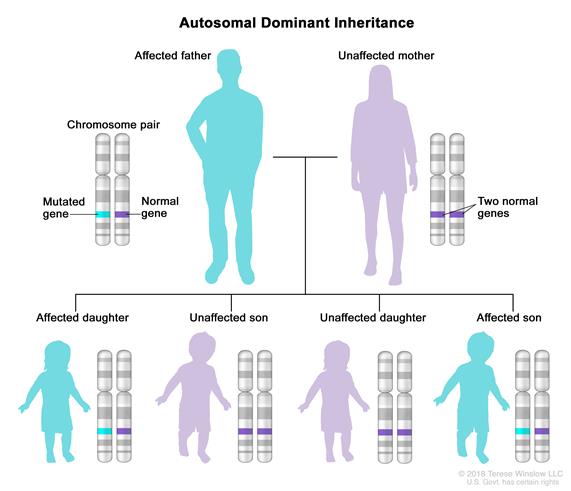
These genes are inherited in an Autosomal Dominant manner.
In an autosomal dominant disorder, the mutated gene is a dominant gene located on one of the nonsex chromosomes (autosomes). You need only one mutated gene to be affected by this type of disorder. A person with an autosomal dominant disorder — in this case (above), the father — has a 50 percent chance of having an affected child with one mutated gene (dominant gene) and a 50 percent chance of having an unaffected child with two normal genes (recessive genes).
Each child of an individual with susceptibility to infection-induced acute encephalopathy 3 (IIAE3) / ANE1 has a 50% chance of inheriting the genetic mutation. Penetrance is incomplete (this means that you may have the gene but never have ANE). Forty percent of people with the mutation for RANBP2 mutation will manifest an episode of acute necrotizing encephalopathy (ANE); 50% of such episodes occur before age two years. A first episode becomes less likely with age, but can occur into adulthood.
OTHER GENES ASSOCIATED WITH ANE –
CPT2

https://www.ncbi.nlm.nih.gov/pubmed/20934285
https://www.jocn-journal.com/article/S0967-5868(18)31725-9/fulltext
HLA Genotypes
https://www.nature.com/articles/gene201632
ribonuclease inhibitor (RNH1)

In summary, the clinical, genetic, and neuroradiological data in this study provide evidence that biallelic variants in RNH1 confer a variably penetrant predilection to an acute viral/febrile encephalopathy that overlaps ANE and ANE1; because of salient distinguishing features, it likely represents a recognizable subtype, and we propose it be referred to as ANE2.
Biallelic variants in ribonuclease inhibitor (RNH1), an inflammasome modulator, are associated with a distinctive subtype of acute, necrotizing encephalopathy.
It is suspected that other, unknown genetic variations are yet to be discovered as a contributing factor of ANE.
Very rare cases of recurrent ANE without RANBP2 missense mutations have also been reported.
Familial Acute Necrotizing Encephalopathy: Evidence from Next Generation Sequencing of Digenic Inheritance
What’s in a name? –
The term “acute necrotizing encephalopathy” (ANE) continues to be used for ANE that is sporadic (i.e., a single occurrence in a family of unknown cause).
Infection-induced acute encephalopathy 3 (IIAE3) is reserved for ANE with a documented RANBP2 pathogenic variant.
The infection-induced acute encephalopathy (IIAE) series describes genetic predispositions to CNS dysfunction following infections:
de novo mutation –
A genetic alteration that is present for the first time in one family member as a result of a variant (or mutation) in a germ cell (egg or sperm) of one of the parents, or a variant that arises in the fertilized egg itself during early embryogenesis. Also called de novo variant, new mutation, and new variant.